Highlights:
- Another DRDO-manufactured drug has been approved by DCGI
- This drug is called ‘2 deoxy D Glucose’
- In the trial, patients’ oxygen needs were greatly reduced
The drug is being developed by DRDO’s Institute of Nuclear Medicine and Alliance Sciences (INMAS) as well as the Hyderabad Center for Cellular and Molecular Biology (CCMB). This drug is currently called ‘2 deoxy D Glucose’. The Hyderabad-based Dr. is responsible for the production of this drug. Given to Reddy Lab.
DCGI approves emergency use of anti-covid drug developed by DRDO
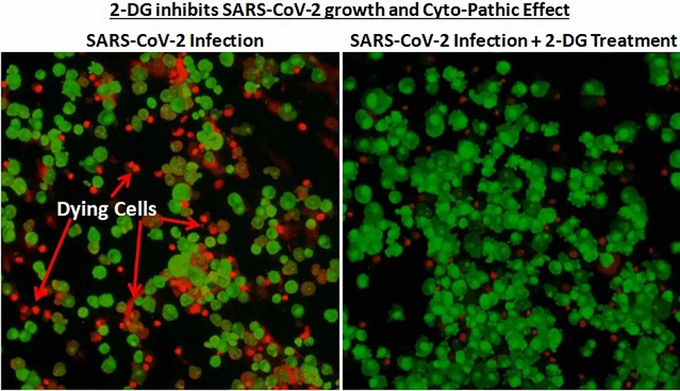
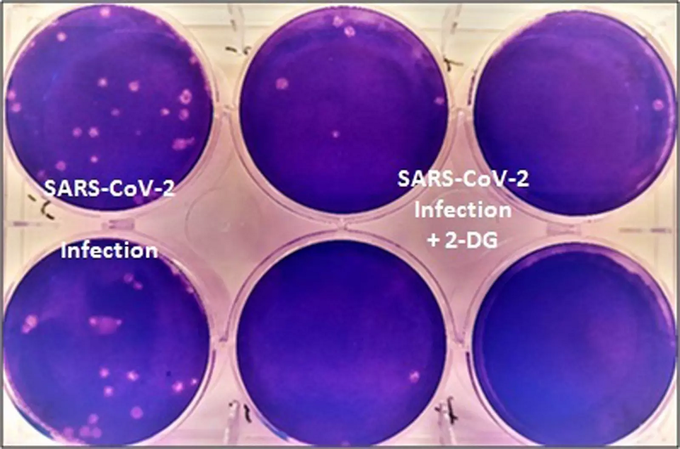
This drug has been successful in clinical trials. Experts claim that the patients on whom the drug was used recovered from the disease as soon as possible. At the same time, it was found that the drug’s need for oxygen was greatly reduced.
Corona patients are less likely to respond to this drug than other coronary artery disease patients. The corona report of these patients is coming ‘negative’ in a very short time. Of course they are recovering quickly.
The drug was first tested in a lab in April 2020 by DRDO scientists. The drug has been shown to be effective in preventing coronavirus infection. On this basis, DCGI had in May 2020 approved the trial of the second phase of the drug.
DCGI approval for emergency use of anti-covid drug developed by DRDO
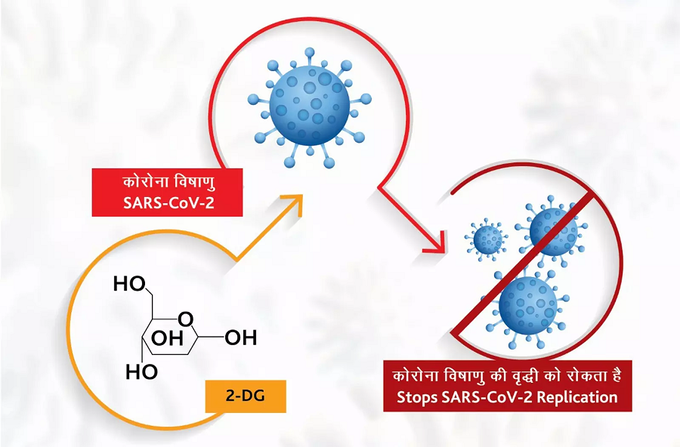
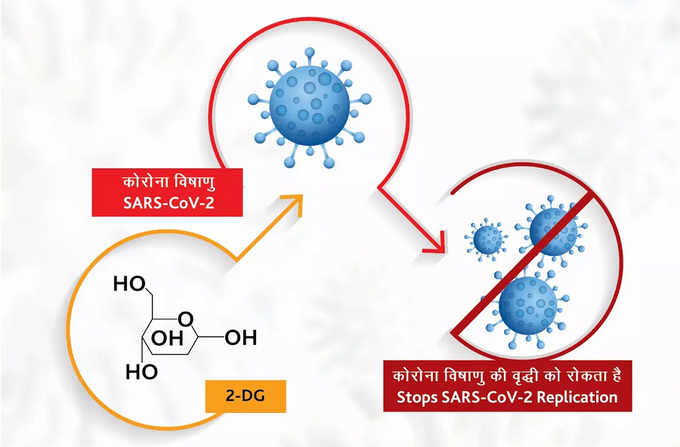
How does ‘2DG’ medicine work?
This powder is given in the form of water mixed with water and given to the patient. The drug accumulates in infected cells. Therefore, it is used to prevent the spread of the virus. This drug is useful in detecting infected cells and controlling the virus. This medication can reduce the patient’s stay in the hospital.
Approval of DRDO medicine
Trial of ‘2DG’
The second trial of the drug was conducted in hospitals across the country. 110 patients from 11 hospitals were included in the trial. The trial took place from May to October.
The third phase was completed in 27 hospitals across the country from December 2020 to March 2021. This included 220 patients. On the third day, 42% of the patients on whom the drug ‘2DG’ was administered ended their dependence on oxygen. The trial was conducted in Maharashtra, Delhi, Uttar Pradesh, Bengal, Gujarat, Rajasthan, Andhra Pradesh, Telangana, Karnataka and Tamil Nadu.
[ad_2]
Source link













 Khan Chacha Restaurant: Oxygen black market in ‘Khan Chacha Restaurant’, owner Navneet Kalra absconding
Khan Chacha Restaurant: Oxygen black market in ‘Khan Chacha Restaurant’, owner Navneet Kalra absconding Covid19: Not to mention the second wave of corona, more than 4 lakh patients per day
Covid19: Not to mention the second wave of corona, more than 4 lakh patients per day INS Vikramaditya: Fire on INS Vikramaditya, no damage
INS Vikramaditya: Fire on INS Vikramaditya, no damage Horrible situation in UP; The system was shaken by the discovery of several bodies in the Yamuna
Horrible situation in UP; The system was shaken by the discovery of several bodies in the Yamuna India China: Foreign Minister boycotts UNSC meeting chaired by China
India China: Foreign Minister boycotts UNSC meeting chaired by China Arvind Kejriwal: Delhi may stay away from the third wave of corona, but …: Arvind Kejriwal
Arvind Kejriwal: Delhi may stay away from the third wave of corona, but …: Arvind Kejriwal






