[ad_1]
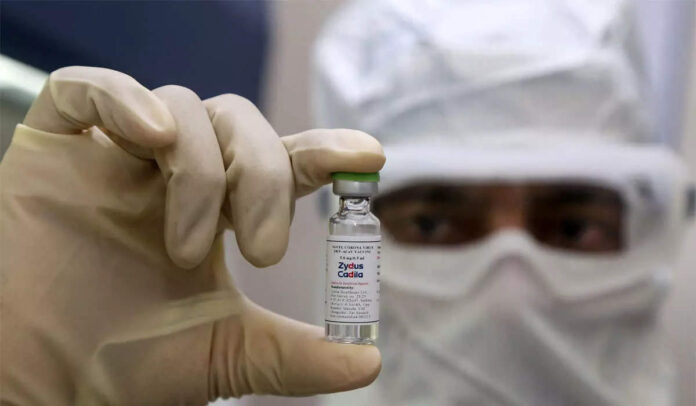
This information was given by the Ministry of Science and Technology, ANI reported on Friday. DCGI approved the world’s first corona vaccine based on the DNA of Zydus Cadillac. The vaccine will be given to children and adults over 12 years of age.
There will be 3 doses of vaccine
3 doses of Zydus Cadillac lees will be given. The largest clinical trial of the vaccine was conducted at more than 50 centers in India. The Ahmedabad-based pharmaceutical company had on July 1 applied to DCGI for emergency approval of the vaccine.
The 6th Corona vaccine will be available in India
A total of six vaccines have been approved in the country so far with the approval of the Zydus Cadillac Corona vaccine. The vaccines for Kovishield, Kovacin, Sputnik V, Moderna and Johnson & Johnson had earlier been approved by the central government.
‘Delta variant’ still dangerous after full vaccination, ICMR study reveals
The Zydus cadilla vaccine is the world’s first DNA-based vaccine. These vaccines inject genetically engineered plasmids into the body. This causes the body to produce corona spike proteins and antibodies to fight off the virus. Most vaccines for coronavirus are of these two doses. But the Zydus cadilla vaccine is three doses.
coronavirus antigen test: anxiety about the third wave of coronavirus; Center on Rapid Antigen Testing Kit Exports
The vaccine is given by a special injector, not by a needle. This does not cause much pain during vaccination. The vaccine also has fewer side effects, the company claims.
[ad_2]
Source link